Absolute measurement of absorption and scattering coefficients
spectra of a multiply scattering medium
Laboratory for Fluorescence Dynamics, Department of Physics, University
of Illinois at Urbana- Champaign, 1110 West Green Street, Urbana, Illinois
61801-3080
* Permanent Address:Istituto di Elettronica Quantistica-Consiglio
Nazionale delle Ricerche, via Panciatichi 56/30, 50127 Firenze, Italy
ABSTRACT
On the basis of the diffusion theory model, frequency-domain spectroscopy
allows for a quantitative determination of the absorption (ua) and
scattering (us') coefficient spectra of a homogeneous multiply scattering
medium. We performed measurements using an intensity modulated light emitting
diode (LED) as the light source. The LED's spectral distribution permits
the study of a spectral region extending for about 80 nm. Data sets (phase
shift and average intensity) at two different source-detector distances
are acquired: the absorption and scattering coefficient spectra of the
medium are then calculated from analytical expressions for ua and us'.
Methylene blue (peak absorption wavelength 656 nm) is used as a test absorbing
material. The methylene blue is dissolved in an aqueous Liposyn solution
which serves as the multiply scattering medium. The relative amounts of
absorber and scatterer are chosen such that the values of ma and ms' match
typical values in tissues. The results obtained for ua(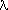 )
with this LED based technique are in quantitative agreement with those
obtained with a standard spectrophotometer in a non- scattering regime.
)
with this LED based technique are in quantitative agreement with those
obtained with a standard spectrophotometer in a non- scattering regime.
1. INTRODUCTION
The determination of the optical properties of tissues is important in
many fields of medicine, both for tissue diagnostics and the monitoring
of physiologically important processes [1-5].
In particular, the measurement of the concentration of a chromophore in
biological tissues can provide useful information. A spectroscopic study
of tissues is complicated by the fact that they are multiply scattering
media, and thus the attenuation of light is due both to absorption and
scattering. The problem of separating the effects of absorption and scattering
has been treated using steady state [6-8],
and time resolved techniques (in both the time-domain [9]
and frequency-domain [10,11]).
We have performed measurements in the frequency-domain, in which the intensity
of the light source is modulated at radio frequency (typically at tens
to hundreds of megahertz), and the detected quantities are the average
intensity (DC component), the amplitude of the intensity oscillation (AC
component), and the phase shift of the detected light relative to the exciting
signal (Ph). A model based on the diffusion approximation to the Boltzmann
transport equation has been developed [12].
It provides expressions for the measured quantities (DC,AC,Ph) in terms
of the optical parameters of the medium, which are the absorption coefficient
(ua), the transport scattering coefficient (us') and the
index of refraction (n). This model is valid for a homogeneous medium
in a multiply scattering regime and far from sources or boundaries. We
used a light emitting diode (LED) as the light source, and we developed
an experimental protocol, based on a two-distances measurement, which allows
for the determination of ua and us' from analytical expressions.
The measurable ranges of ua and us', as well as the sensitivity
of this method to changes in ua and us', are discussed.
2. METHODS
The medium we have studied is an aqueous solution of Liposyn III 20% (an
intravenous fat emulsion) from Abbott Laboratories, in which a test absorbing
material (methylene blue) was dissolved. The concentration of Liposyn,
which provided the scattering material, was chosen to be 7.7% by volume
(i.e. solids content of 1.54%) yielding a medium scattering coefficient
on the order of 20 (1/cm), which is typical for soft tissues [13].
Methylene blue has an absorption spectrum peaked at 656 nm and an extinction
coefficient of 182 (1/(cm*mM)) at 664 nm [14].
We used several concentrations of methylene blue, from 0 to 0.450 uM, in
order to cover a typical range of values of ua in tissues (0.01
to 0.1 1/cm) [13]. The measurements were conducted
in a 2.3 l cylindrical container whose dimensions were 9 cm in height
and 18 cm in diameter.
As the light source, we utilized a light emitting diode (LED)
manufactured by Hewlett Packard (part number: HLMP-4101). We modulated
the LED's intensity by applying a sinusoidal voltage generated by a frequency
synthesizer (Marconi Instruments, Model 2022A) and amplified by an ENI
Model 403 LA RF Amplifier. In general, a frequency domain measurement is
more accurate at higher modulation frequencies, so we looked for the highest
frequency of the sinusoidal voltage supply which provided a good AC signal
at the LED's output. This frequency was 60 MHz for the LED we used, and
the peak to peak supplied voltage was 10 V. The negative front of the sinusoidal
voltage was less than the reverse breakdown voltage of the LED (15 V).
In these working conditions the LED's output was characterized by a peak
wavelength of 665 nm, a spectral line fullwidth at half maximum of about
30 nm, a total power of about 0.2 mW, and a modulation ratio (AC/DC) of
60%. Its spectral emission enabled us to accomplish measurements at wavelengths
ranging from 620 to 700 nm, which is appropriate for measuring the spectrum
of methylene blue (lmax = 656 nm).
The multiply scattered light was detected by a bundle of glass
optical fibers of overall diameter of 3 mm. This bundle was positioned
at a distance r from the light source, and its output was dispersed
via a monochromator (10 cm ISA Instrument SA, Inc.) and detected by the
sample photomultiplier tube (PMTs). The spectral resolution was 4 nm. The
detection system is a typical one for frequency domain measurements, and
is based on a well established cross-correlation technique [15,16].
The cross-correlation frequency was set to 80 Hz. We referenced the values
of DC, AC and phase detected by the sample PMT (PMTs) to those detected
by the reference PMT (PMTr) which collected a signal from an optical fiber
in close contact with the LED (both PMT's are manufactured by Hamamtsu
(Japan), model R928). This procedure provided a correction for variations
in the LED's output characteristics that might have occured during the
measurement. The experimental setup is shown in Fig. 1
.

Fig. 1. Experimental setup.
-
2.2. Measurement protocol
The diffusion approximation to the Boltzmann transport equation provides
analytical expressions for the frequency-domain parameters as a function
of the absorption coefficient (ua), the transport scattering coefficient
(us'), and the index of refraction (n) of the medium [12].
These expressions, which are valid for a homogeneous infinite medium at
points which are far from sources and boundaries, predict a linear dependence
of Ph, ln(rUdc) and ln(rUac) on r (where
r is the source-detector separation, Udc is the DC component
of the photon density, Uac is the amplitude of the AC component
of the photon density). If we consider two different source detector separations,
let's say r1 and r2 with r1< r2, we can
define the following quantities:
.
Finally, making the further assumption that w/2p<
< cus'/n (where w/2p
is the modulation frequency, and c is the speed of light in vacuum)
we can write [12]:
(1)
(2)
(3)
We observe that the condition w/2p<
< cus'/n holds for most biological tissues in the red-near
IR spectral region for modulation frequencies up to 1 GHz. The idea is
then to perform two measurements at two different source-detector separations
(r1 and r2) from which to obtain dc, ac, ph.
We point out that the source terms do not appear in Eqs. 1-3, so that dc,
ac, ph depend only on the modulation frequency and on the
optical parameters of the medium. Furthermore, the spectral instrument
response factor cancels in dc and ac because they contain the ratio of
Udc and Uac measured at different source-detector separations,
but at the same wavelength. Once dc, ac, ph are measured
ua and us' can be calculated from Eqs. 1-3. Inspection of
these equations shows that they are not independent since they contain
only two unknowns, i.e. ua/n and n(ua+us'). If n is
known, one can choose to obtain ua and us' from three different
sets of measurements: dc and ph (DC and phase), ac
and ph (AC and phase), dc and ac (DC and AC). The
analytical expressions for ua and us' in the three cases
are given in Table 1.
On the basis of these expressions, the errors on ua and us'
can be evaluated from the estimates of the errors on dc, ac,
ph. The result is that, for typical values of the parameters in
tissues, the errors on ua and us' in the (dc,ac)
measurement scheme are much larger than those in the (dc,ph)
and (ac,ph) schemes. This is due to the fact that the values
of dc and ac are very close, differing by no more than a
few percent. We chose to use the (dc,ph) scheme because the
error on the DC signal is typically smaller than the error on the AC signal.
The use of DC and phase in frequency-domain measurements was also suggested
as an alternative to a phase and modulation (AC/DC) measurement [10]
In summary, our measurement protocol consists of acquiring data
at two source-detector distances (r1, r2) and calculatedc
and ph. From the expressions relative to the (dc,ph)
measurement scheme in Table 1, we obtain the values of ua and us'.
This procedure, applied wavelength by wavelength within the whole spectral
region covered by the LED (620 to 700 nm) provides the spectra of ua
and us'. The choice of the values of r1 and r2 (r1<
r2) relies on two considerations:
-
r1 must be greater than the photon mean free path in order for Eqs.
1-3 to be valid;
-
r2 must allow for an acceptable detected light signal: since the
density of photons decays exponentially with distance, this condition constitutes
an upper limit for r2.
We chose r1 to be 1.5 cm, while r2 ranged from 4.0 to 2.5
cm depending on the amount of absorber in the medium.
3. RESULTS
First we performed a control measurement on the aqueous Liposyn solution
alone. In the absence of methylene blue we expect to measure essentially
the absorption spectrum of water, while the scattering spectrum is related
to the suspended particles of Liposyn. In Fig. 2 we
show the spectra we measured for ua and us'. The spectrum
of ua is compared to values of ua for water measured at several
wavelengths by Hale and Querry [17].
The order of magnitude of ua is the same for both quantities and
their spectral dependence is qualitatively comparable. We attribute the
deviations from the literature values of ua for water to a contribution
of the Liposyn to absorption, and to boundary effects due to the finite
size of the container we used. The spectrum of us' is compared to
the curve us'(l) predicted by van Staveren
et al. on the basis of Mie theory calculations for 1.54% Intralipid [18].
Even if van Staveren considered a slightly different medium, we observe
that the values of us' and the spectral dependence are essentially
reproduced.

Fig. 2. Scattering (solid line) and absorption (dashed line)
coefficients spectra of the Liposyn solution containing no methylene blue.
The two spectra are compared respectively with us'(l)
predicted by van Staveren et al. (dotted line), and with several values
of ua(l) for water known in literature
(symbols).
Next we measured the absorption spectra for different concentrations
of methylene blue in the Liposyn solution. We subtracted the absorption
spectrum of the Liposyn solution containing no methylene blue to obtain
the corrected absorption spectra for methylene blue alone. The results
obtained with our measurement protocol were then compared to the absorption
spectra obtained with a spectrophotometer (Perkin Elmer LAMBDA 5) for the
same concentrations of methylene blue in a non scattering solution. The
comparison, reported in Fig. 3 for three methylene
blue concentrations, shows a quantitative agreement between the measurements
in the multiply scattering regime (performed via the frequency-domain LED
technique) and the measurements in minimal scattering regime (performed
by a standard spectrophotometer). We have also analyzed the dependence
of ua and us' on methylene blue concentration. The result
is shown in Fig. 4 for l=680
nm: us' is essentially unaffected by the concentration of absorber,
while ua shows a linear dependence. We observe that our results
have been obtained by assuming that n=1.33 (which is the index of
refraction of water in the spectral region considered). The good quantitative
results we found justify such a choice for n.

Fig. 3. Quantitative comparison between methylene blue absorption
coefficient spectra measured in the strongly scattering medium by the LED
technique (symbols) and in a non-scattering regime by a spectrophotometer
(lines). The spectra correspond to MB concentrations of 0.090, 0.225, and
0.450 uM as labeled. Errors bars for the experimental data relative to
the strongly scattering medium are shown every 20 nm.
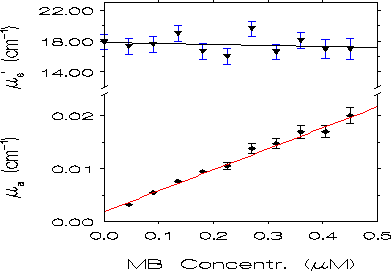
Fig. 4. Dependence of the values of ua and us' at 680 nm on
methylene blue concentration. The straight lines are obtained by a weighted
least square method. When the error bars are not displayed they are on
the order of the symbol dimensions.
4. DISCUSSION
The range of values for ua and us' that can be measured with
the frequency-domain LED method is determined by the following conditions:
-
in order for the diffusion model to be valid, us' has to be much
greater than ua (let's say us'> 20ua);
-
since the error on ph is on the order of tenths of a degree, we
want ph to be at least 1 deg;
-
an upper limit to ua and us' is given by the amount of light
signal at the greater distance (r2). We assumed that the DC and
AC signals at r2 are not smaller than 1/300 times the signals at
r1.
By imposing these conditions we can evaluate the ranges of measurable values
of ua and us' using our instrument. The result is shown on
a us'-ua log-log diagram in Fig. 5 ,
where one can see that the typical values of ua and us' in
tissues fall inside the measurable region of the us'-ua plane.
The sensitivity of this technique to changes in the optical parameters
can be evaluated by the relative errors on ua and us'. These
errors are typically within a few percent.
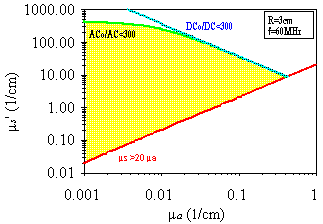
Fig. 5. Values of ua and us' that can be measured with our
instrument the frequency-domain LED technique. The conditions discussed
in the text define the measurable region in the us'-ua plane, which is
shown shaded. Note that the typical values of ua and us' in tissues (ua~0.01-0.1
1/cm, us'~1-100 1/cm) fall inside the measurable region.
This work has dealt with a macroscopically homogeneous strongly scattering
medium in a quasi-infinite geometry. Of course, the application of this
technique to in vivo spectroscopy of tissues requires some further
developments. First, biological tissues are not as homogeneous as an aqueous
Liposyn solution. Second, they do not provide a quasi-infinite geometry
(at least for non-invasive applications). The first point has already been
considered, [9 ,19]
and the reasonable results obtained in vivo on the basis of homogeneous
medium models are encouraging. The second point requires the use of different
boundary conditions. A first approach might be that of a semi-infinite
geometry with source and detector placed on the surface. Although the diffusion
approximation is not valid in such a geometry, it still constitutes a reasonable
starting point [9]. We are currently working on this
subject.
Several characteristics of the LED technique make it attractive
from a practical standpoint:
-
the instrumentation required by the LED technique can be made compact and
portable;
-
frequency-domain methods allow for fast acquisition times (~10 ms) which
can provide real time measurements. Monitoring of fast dynamic processes
is then feasible;
-
the total power emitted by LEDs is typically less than a few mW and is
distributed over a wide solid angle. This properties make LEDs safe for
medical applications;
-
the LED method is cost effective, especially when compared to other spectroscopic
techniques.
5. ACKNOWLEDGMENTS
This work was performed at the Laboratory for Fluorescence Dynamics at
the University of Illinois at Urbana- Champaign (UIUC), which is supported
by the National Institutes of Health (NIH), grant RR03155 and by UIUC.
This research is also supported by grant CA57032 from the NIH. The authors
thank Julie Butzow for help in preparing this paper.
6. REFERENCES
-
G. Wagnieres, C. Depeursinge, P. Monnier, M. Savary,
P. Cornaz, A. Chatelain, and H. van den Bergh, "Photodetection of Early
Cancer by Laser Induced Fluorescence of a Tumor-Selective Dye: Apparatus
Design and Realization," Proc. SPIE - Int. Soc. Opt. Eng. (USA) 1203,
43-52 (1990). back
-
M. S. Patterson, B. C. Wilson, J. W. Feather, D. M. Burns, and W. Pushka,
"The Measurement of Dihematopotphyrin Ether Concentration in Tissue by
Reflectance Spectrophotometry," Photochem. Photobiol. 46, 337-343
(1987). back
-
M. Cope and D. T. Delpy, "System for Long-Term Measurement of Cerebral
Blood and Tissue Oxygenation on Newborn Infants by Near Infrared Transillumination,"
Med. Biol. Eng. Comput. 26, 289-294 (1988). back
-
B. C. Wilson and M. S. Patterson, "The Physics of Photodynamic Therapy,"
Phys. Med. Biol. 31, 327- 360 (1986). back
-
S. L. Jacques and S. A. Prahl, "Modeling Optical and Thermal Distribution
in Tissue During Laser Irradiation," Laser Surg. Med. 6, 494-503
(1987). back
-
F. F. Jobsis, J. H. Keizer, J. C. LaManna, and M. Rosenthal,
"Reflectance Spectrophotometry of cytochrome aa3 in vivo,"
J. Appl. Physiol. 43, 858-872 (1977). back
-
P. Parsa, S. L. Jacques, and N. S. Nishioka, "Optical Properties of Rat
Liver Between 350 and 2200 nm," Appl. Opt. 28, 2325-2330 (1989).
back
-
T. J. Farrel, M. S. Patterson, and B. Wilson, "A Diffusion Theory Model
of Spatially Resolved, Steady-State Diffuse Reflectance for the Noninvasive
Determination of Tissue Optical Properties in vivo," Med. Phys.
19, 879-888 (1992). back
-
M. S. Patterson, B. Chance, and B. C. Wilson, "Time
Resolved Reflectance and Transmittance for the Noninvasive Measurement
of Tissue Optical Properties," Appl. Opt. 28, 2331-2336 (1989).
back
-
M. S. Patterson, J. D. Moulton, B. C. Wilson, K. W. Berndt, and J. R. Lakowicz,
"Frequency-Domain Reflectance for the Determination of the Scattering and
Absorption Properties of Tissue," Appl. Opt. 30, 4474-4476 (1991).
back
-
J. B. Fishkin, E. Gratton, M. J. van de Ven, and W. W. Mantulin, "Diffusion
of Intensity Modulated Near-IR Light in Turbid Media," in Time Resolved
Spectroscopy and Imaging of Tissues, B. Chance editor, Proc. SPIE 1431,
122-135 (1991). back
-
J. B. Fishkin and E. Gratton, "Propagation of Photon-Density
Waves in Strongly Scattering Media Containing an Absorbing Semi-Infinite
Plane Bounded by a Straight Edge," J. Opt. Soc. Am. A 10, 127-140
(1993). back
-
W. F. Cheong, S. A. Prahl, and A. J. Welch, "A Review
of the Optical Properties of Biological Tissues," IEEE J. Quantum Electron.
26, 2166-2185 (1990). back
-
Aldrich Chemical Company Laboratories, Milwaukee,
Wisconsin (1993). back
-
R. D. Spencer and G. Weber, "Measurement of Subnanosecond
Fluorescence Lifetime with a Cross-Correlation Phase Fluorometer," Ann.
N. Y. Acad. Sci. 158, 361-376 (1969). back
-
B. A. Feddersen, D. W. Piston, and E. Gratton, "Digital Parallel Acquisition
in Frequency Domain Fluorometry," Rev. Sci. Instrum. 60, 2929-2936
(1989). back
-
G. M. Hale and M. R. Querry, "Optical Constants
of Water in the 200 nm to 200 um Wavelength Region," Appl. Opt. 12,
555-563 (1973). back
-
H. J. van Staveren, C. J. M. Moes, J. van Marle, S. A. Prahl, and M. J.
C. van Gemert, "Light Scattering in Intralipid-10% in the Wavelength Range
of 400-1100 nm," Appl. Opt. 30, 4507-4514 (1991). back
-
E. M. Sevick, B. Chance, J. Leigh, S. Nioka, and
M. Maris, "Quantitation of Time- and Frequency-Resolved Optical Spectra
for the Determination of Tisuue Oxigenation," Anal. Biochem. 195,
330-351 (1991). back
Back to top
