The Daily Free Press recounts the HUBWeek event in which Center Director Bruce Rosen and medical illustrator Danny Quirk spoke about the intersectionality of human anatomy and visual art.
When Researchers Work Together
 |
|
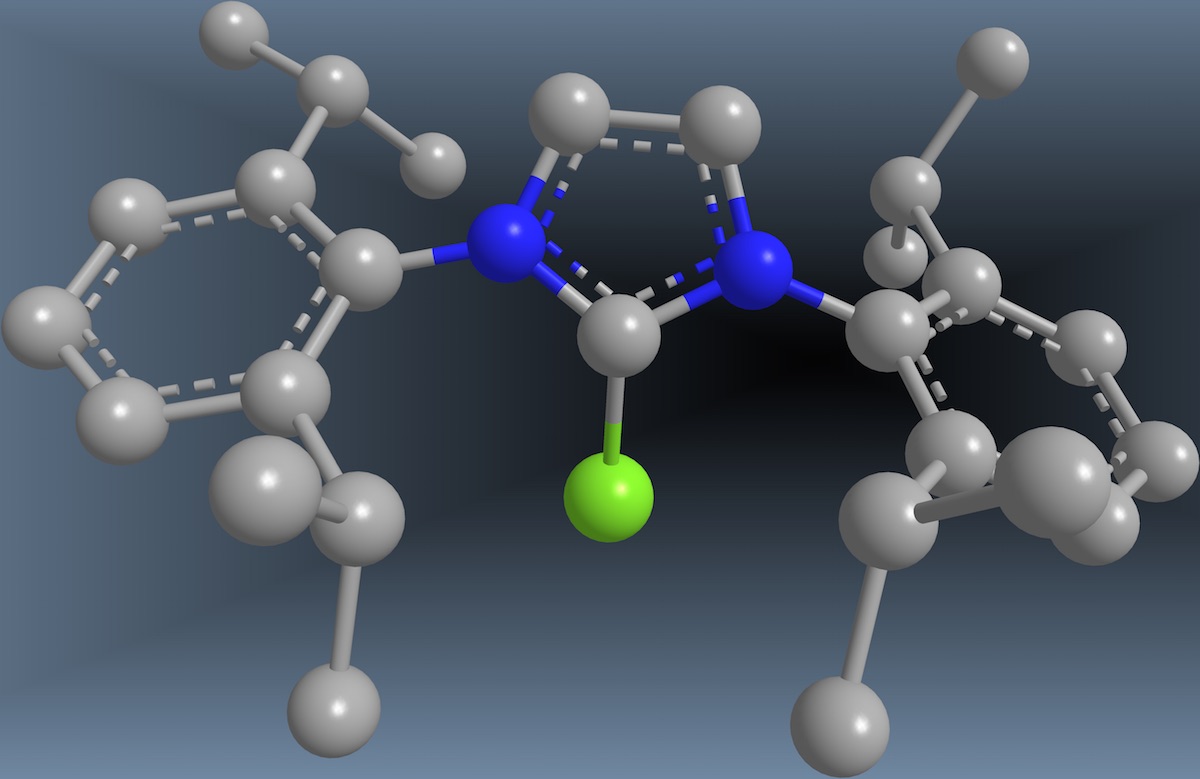
In a recent Nature study, chemists Jacob Hooker and Tobias Ritter evidenced the value of collaboration in academic research. |
In a Nature paper published online last month the Martinos Center’s Jacob Hooker and colleagues at Harvard University, Tobias Ritter and Connie Neumann, took an important step forward in fluorine-18 chemistry, reporting a new reaction that could help to expand significantly the number of radiotracers available for PET imaging. The potential benefits of this are many and profound. They include improvements in diagnosis and assessment of patients’ responses to drugs as well as advances in our ability to understand the biology in a range of diseases and disorders.
As is always the case in academic research, the success of the study was due to a number of factors aligning in just the right way. Among these, of course, are the initial insight—the indefinable “aha” moment that shows you the way forward in tackling a given problem—and the resources needed to act upon that insight. But the work also owes a debt to another, perhaps less remarked upon aspect of research: that is, the time-honored tradition of collaboration.
The Nature study builds upon a longstanding collaboration between Hooker and his group at the Martinos Center and the laboratory of Tobias Ritter, which was originally based in Harvard’s Department of Chemistry and Chemical Biology. Each of the two groups brings to the collaboration extensive experience in a particular area of fluorine chemistry, with capabilities the researchers knew would prove complementary in tackling the task at hand.
“When we met, Tobias and his lab were making huge leaps in our understanding of fluorine chemistry and how to control it through catalysis,” said Hooker, Director of Radiochemistry at the Martinos Center and an associate professor of radiology at Harvard Medical School. “The synergy between the Ritter lab's fundamental advances in fluorine chemistry and my lab's ability to apply the new knowledge in radiotracer chemistry for PET imaging was obvious and incredible.”
The two investigators were introduced by Greg Sorensen, former co-Director of the Martinos Center, following a discussion between Sorensen and Hooker over a cup of hot chocolate in Harvard Square. Ritter had previously developed a reagent—based on palladium—that he knew might facilitate development of fluorine-18 radiotracers, an important goal in PET imaging. But he wasn’t yet sure how he would get to that point. This is where Hooker and his group came in.
Less than two years later the collaboration bore its first real fruit: a Science paper describing a reagent that enables synthesis of typically unavailable radiotracers by way of late-stage fluorination. The paper made a considerable splash when it was published, upending the conventional wisdom about what is possible with fluorination and opening an array of potential radiochemistry applications. In the five years since, it has been cited more than 200 times.
The work served to bridge the growing gap between research in the organic and radiochemistry communities. Just as important, though, it offered a model for bridging the communities themselves. As radiochemistry has emerged as a discipline in its own right, it has grown ever closer to medical imaging, in a sense leaving the company of the more “traditional” areas of chemistry. Today, you will find few radiochemistry programs in universities; they mostly exist as pockets in hospitals or national labs. The Science paper marked a turning point. In the wake of the study, we are seeing more collaborations between organic or organometallic chemists and radiochemists, and university chemistry departments are starting to take another look at the possibilities of medical imaging.
The Science paper represented a major conceptual advance, but there were still some practical limitations in the approach described. In the wake of the study, Hooker and Ritter and their respective groups set to work in addressing these. Their efforts ultimately led to the recently published Nature paper, in which they describe a new reaction, based in part on the PhenoFluor reagent, that makes possible a range of new PET radiotracers and radiochemistry applications.
In a recent conversation Hooker reflected on the reasons for which this longstanding collaboration has gone as well as it has. In doing so, he identified several key factors he feels have contributed to its success. First, he said, is an open-door policy and associated logistical strategies facilitating access to one another for collaborating members of the labs. This included Hooker and Ritter co-mentoring postdoctoral fellows and graduate students. “We recognized very early that scientific exchange comes from people physically and mentally operating in two environments,” he continued. “I do not think the collaboration would have been nearly as successful if we operated with a wall between us.”
Recognizing one another’s contributions is also essential. Though Hooker and Ritter are both organic chemists by training, they have expertise in different domains and “fully embrace the brain-power of one another, deferring at critical moments to the decisions of the ‘right’ expert,” Hooker said. This ensures that the best decisions are made at any given juncture, but also helps to create an environment in which the investigators gain ever deeper understandings of the others’ areas of expertise. “Over time we've each learned a lot more about one another's domain and can now communicate really fluently.”
Ultimately, simply maintaining an atmosphere of trust and mutual respect can prove advantageous. In the case of this collaboration, doing so has enabled the investigators to work through research-related issues or interpersonal / inter-lab challenges as they arise and quickly move on.
Ritter has since left Harvard; last year he accepted an offer to become a director of the Max Planck Institute in Muelheim, Germany. Even so, he and Hooker have continued their collaboration, addressing practical challenges found in the Nature study. Some of the logistics have necessarily changed, or at least will change as the work continues. But the synergy remains, Hooker said. No matter where the whims of time may take them, the investigators will stay colleagues and friends, thanks in large part to the care and attention they have devoted to building a strong collaborative effort.
“Concerted nucleophilic aromatic substitution with 19F− and 18F−,” by Constanze N. Neumann, Jacob M. Hooker and Tobias Ritter, was published on the Nature website on May 18, 2016.


